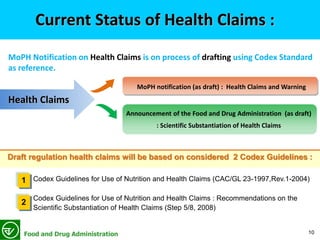
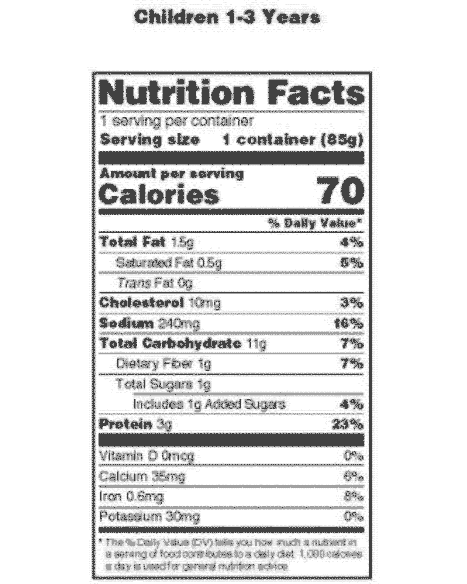
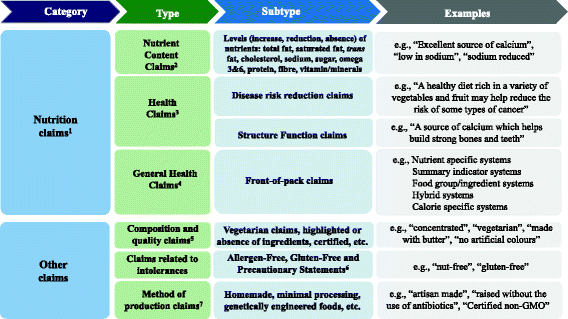
42 health claims on food labels are standardized and regulated
en.wikipedia.org › wiki › Nutrition_facts_labelNutrition facts label - Wikipedia The Ministry of Health and Family Welfare had, on September 19, 2008, notified the Prevention of Food Adulteration (5th Amendment) Rules, 2008, mandating packaged food manufacturers to declare on their product labels nutritional information and a mark from the F.P.O or Agmark (Companies that are responsible for checking food products) to enable ... Page: Journal of Allergy and Clinical Immunology Sep 13, 2022 · An official publication of the American Academy of Allergy, Asthma, and Immunology, The Journal of Allergy and Clinical Immunology brings timely clinical papers, instructive case reports, and detailed examinations of state-of-the-art equipment and techniques to clinical allergists, immunologists, dermatologists, internists, and other physicians concerned with clinical manifestations of ...
› en › health-canadaFront-of-package nutrition symbol labelling guide for industry Nutrition and health-related statements and claims may be made on the label or in advertisements for foods on a voluntary basis. However, when they are made, they must comply with subsection 5(1) of the Food and Drugs Act, the food provisions of the FDR, and subsection 6(1) of the Safe Food for Canadians Act and should follow any applicable ...

Health claims on food labels are standardized and regulated
› food › information-consumers-usingQuestions and Answers on Dietary Supplements | FDA May 06, 2022 · Among the claims that can be used on dietary supplement labels are three categories of claims that are defined by the FD&C Act and FDA regulations: health claims (claims about the relationship ... abcnews.go.com › healthHealth News | Latest Medical, Nutrition, Fitness News - ABC ... Get the latest health news, diet & fitness information, medical research, health care trends and health issues that affect you and your family on ABCNews.com en.wikipedia.org › wiki › Dietary_supplementDietary supplement - Wikipedia Definition. In the United States, the Dietary Supplement Health and Education Act of 1994 provides this description: "The Dietary Supplement Health and Education Act of 1994 (DSHEA) defines the term "dietary supplement" to mean a product (other than tobacco) intended to supplement the diet that bears or contains one or more of the following dietary ingredients: a vitamin, a mineral, an herb or ...
Health claims on food labels are standardized and regulated. and Supplements Rooted in Science - Life Extension Get clinically-studied, premium vitamins and supplements and lab tests from the people who’ve spent 40 years passionately pursuing healthy living. en.wikipedia.org › wiki › Dietary_supplementDietary supplement - Wikipedia Definition. In the United States, the Dietary Supplement Health and Education Act of 1994 provides this description: "The Dietary Supplement Health and Education Act of 1994 (DSHEA) defines the term "dietary supplement" to mean a product (other than tobacco) intended to supplement the diet that bears or contains one or more of the following dietary ingredients: a vitamin, a mineral, an herb or ... abcnews.go.com › healthHealth News | Latest Medical, Nutrition, Fitness News - ABC ... Get the latest health news, diet & fitness information, medical research, health care trends and health issues that affect you and your family on ABCNews.com › food › information-consumers-usingQuestions and Answers on Dietary Supplements | FDA May 06, 2022 · Among the claims that can be used on dietary supplement labels are three categories of claims that are defined by the FD&C Act and FDA regulations: health claims (claims about the relationship ...
![PDF] Nutrition labels and health claims: the global ...](https://d3i71xaburhd42.cloudfront.net/f8e40d7b317600da54b78934835b7ec21cd9b57d/24-Table1-1.png)

:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3652082/healthy-choice.0.png)


















![PDF] Nutrition marketing on processed food packages in Canada ...](https://d3i71xaburhd42.cloudfront.net/6273f6117a666d80f88bc9641ac54dbfc8f285c5/2-Table1-1.png)










Post a Comment for "42 health claims on food labels are standardized and regulated"